Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyajima, Yusuke*; Saito, Ayaka*; Kagi, Hiroyuki*; Yokoyama, Tatsunori; Takahashi, Yoshio*; Hirata, Takafumi*
Geostandards and Geoanalytical Research, 45(1), p.189 - 205, 2021/03
Times Cited Count:5 Percentile:21.77(Geochemistry & Geophysics)Uncertainty for elemental and isotopic analyses of calcite by LA-ICP-MS is largely controlled by the homogeneity of the reference materials (RMs) used for normalization and validation. In order to produce calcite RMs with homogeneous elemental and isotopic compositions, we incorporated elements including U, Pb, and rare earth elements into calcite through heat- and pressure-induced crystallization from amorphous calcium carbonate that was precipitated from element-doped reagent solution. X-ray absorption spectra showed that U was present as U(VI) in the synthesized calcite, probably with a different local structure from that of aqueous uranyl ions. The uptake rate of U by our calcite was higher in comparison to synthetic calcite of previous studies. Variations of element mass fractions in the calcite were better than 12% 2RSD, mostly within 7%. The 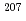 Pb/
Pb/ Pb ratio in the calcite showed
Pb ratio in the calcite showed  1% variations, while the
1% variations, while the  U/
U/ Pb ratio showed 3-24% variations depending on element mass fractions. Using the synthetic calcite as primary RMs, we could date a natural calcite RM, WC-1, with analytical uncertainty as low as
Pb ratio showed 3-24% variations depending on element mass fractions. Using the synthetic calcite as primary RMs, we could date a natural calcite RM, WC-1, with analytical uncertainty as low as  3%. The method presented can be useful to produce calcite with controlled and homogeneous element mass fractions, and is a promising alternative to natural calcite RMs for U-Pb geochronology.
3%. The method presented can be useful to produce calcite with controlled and homogeneous element mass fractions, and is a promising alternative to natural calcite RMs for U-Pb geochronology.
Sato, Junya; Shiota, Kenji*; Takaoka, Masaki*
Journal of Hazardous Materials, 385, p.121109_1 - 121109_9, 2020/03
Times Cited Count:9 Percentile:43.42(Engineering, Environmental)Lead is a hazardous heavy metal that can be stabilized by incorporation into the matrix of aluminosilicate bearing phases as they solidify. The actual mechanism by which lead is stabilized, however, continues to be unclear because the individual mechanisms of Pb incorporation into crystalline and amorphous aluminosilicate phases have not yet been studied separately. A detailed investigation of the incorporation of Pb into the amorphous phase of aluminosilicate solids was therefore performed. Amorphous aluminosilicate solids were synthesized with 0.7, 1.5, and 3.7 wt% of Pb from aluminosilicate gel produced from chemical reagents. Based on Raman spectroscopy, the Si-O stretching vibration bond shifted to lower wavenumbers with increasing Pb concentration. This shift suggested that covalent bonding between Pb and O in the matrix of the aluminosilicate solids increased. In addition, sequential extraction revealed that most of the Pb (75-90%) in the aluminosilicate solids was in a poorly soluble form (i.e. reducible, oxidizable, and residual fractions). These findings indicate that most of Pb is bonded covalently to the amorphous phase in aluminosilicate solids.
 (am) solubility at 25
(am) solubility at 25 C in the Ca
C in the Ca -Na
-Na -H
-H -Cl
-Cl -OH
-OH -H
-H O system; A Critical review
O system; A Critical reviewRai, D.*; Kitamura, Akira; Altmaier, M.*; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Journal of Solution Chemistry, 47(5), p.855 - 891, 2018/05
Times Cited Count:8 Percentile:8.45(Chemistry, Physical)We have critically reviewed experimental data for Zr hydrolysis constant values for formation of several mononuclear and polynuclear species and a solubility product value for ZrO (am). We have determined new/revised values for the formation constants of Zr(OH)
(am). We have determined new/revised values for the formation constants of Zr(OH)
 , Zr(OH)
, Zr(OH) (aq), Zr(OH)
(aq), Zr(OH)
 , Zr(OH)
, Zr(OH)
 and Ca
and Ca Zr(OH)
Zr(OH)
 , and the solubility product for ZrO
, and the solubility product for ZrO (am) after the critical review.
(am) after the critical review.
Kobayashi, Taishi*; Teshima, Takeshi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Nuclear Science and Technology, 54(2), p.233 - 241, 2017/02
Times Cited Count:6 Percentile:51.46(Nuclear Science & Technology)Zr solubility in the presence of gluconic acid (GLU) and isosaccharinic acid (ISA) was investigated as a function of hydrogen ion concentration (pH ) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH
) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH and GLU concentration suggested the existence of Zr(OH)
and GLU concentration suggested the existence of Zr(OH) (GLU)
(GLU)
 in the neutral pH region and Zr(OH)
in the neutral pH region and Zr(OH) (GLU)(GLU
(GLU)(GLU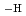 )
) in the alkaline pH region above pH
in the alkaline pH region above pH 10 as the dominant species in the presence of 10
10 as the dominant species in the presence of 10 - 10
- 10 mol/dm
mol/dm (M) GLU. In the presence of ISA, the dominant species Zr(OH)
(M) GLU. In the presence of ISA, the dominant species Zr(OH) (ISA)
(ISA)
 and Zr(OH)
and Zr(OH) (ISA)(ISA
(ISA)(ISA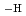 )
) were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH)
were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH) (am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
(am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.

 -edge of perpendicular magnetic Dy
-edge of perpendicular magnetic Dy Co
Co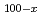 films
filmsAgui, Akane; Mizumaki, Masaichiro*; Asahi, Toru*; Sayama, Junichi*; Matsumoto, Koji*; Morikawa, Tsuyoshi*; Matsushita, Tomohiro*; Osaka, Tetsuya*; Miura, Yoshimasa*
Journal of Alloys and Compounds, 408-412, p.741 - 745, 2006/02
Times Cited Count:7 Percentile:46.38(Chemistry, Physical)no abstracts in English
Zhu, X. D.; Naramoto, Hiroshi; Xu, Y.; Narumi, Kazumasa; Miyashita, Kiyoshi*
Physical Review B, 66(16), p.165426_1 - 165426_5, 2002/10
Times Cited Count:14 Percentile:57.58(Materials Science, Multidisciplinary)no abstracts in English
Naramoto, Hiroshi; Xu, Y.; Narumi, Kazumasa; Vacik, J.; Zhu, X.; Yamamoto, Shunya; Miyashita, Kiyoshi*
Materials Research Society Symposium Proceedings, Vol.647, p.O5.18.1 - O5.18.16, 2001/00
no abstracts in English
Nakata, Yoshiyuki*; Hara, Naoyuki*; Hirotsu, Yoshihiko*; Emura, Shuichi*; Makino, Akihiro*; Uruga, Tomoya*; Harada, Makoto*; Nishihata, Yasuo; Yoneda, Yasuhiro; Kubozono, Yoshihiro*
Japanese Journal of Applied Physics, 38(Suppl.38-1), p.404 - 407, 1999/06
The EXAFS spectra on Zr K-edge are investigated for as-formed Fe-7at%Zr-3at%B amorphous alloys and annealed ones at 693 K and 773 K for an hour in order to reveal local structural changes due to annealing. The analysis shows that the atomic distances of Fe-Zr and Zr-Zr pairs increase during annealing and approach those in a Fe3Zr or/and Fe2Zr metallic comound. The change in the atomic distances suggests that medium range ordered clusters should be formed during annealing.
Toda, Naohiro*; Katayama, Yoshinori; Tsuji, Kazuhiko*
Review of High Pressure Science and Technology, 7, p.647 - 649, 1998/03
The electrical conductivity  has been measured at pressures
has been measured at pressures  to 8 GPa and temperatures
to 8 GPa and temperatures  of 77-300K in evaporated amorphous Ge (a-Ge), a-Ge-Cu alloys and a-Ge-Al alloys. The
of 77-300K in evaporated amorphous Ge (a-Ge), a-Ge-Cu alloys and a-Ge-Al alloys. The  dependence of
dependence of  is well described by a power lw at low temperatures below 150 K, which is expemcted from a multi-phonon tunneling transition process model with weak electron-lattice coupling, rather than the Mott's variable range hopping conduction model. The exponent
is well described by a power lw at low temperatures below 150 K, which is expemcted from a multi-phonon tunneling transition process model with weak electron-lattice coupling, rather than the Mott's variable range hopping conduction model. The exponent  in the power law changes with increasing pressure. For both a-Ge
in the power law changes with increasing pressure. For both a-Ge Cu
Cu and a-Ge
and a-Ge Al
Al alloys, d(ln
alloys, d(ln  )/d
)/d show positive values in the low pressure region and negative values in the high pressure region. Results are discussed from several hopping conduction models.
show positive values in the low pressure region and negative values in the high pressure region. Results are discussed from several hopping conduction models.
*; *; Fukumura, H.*; *; Nishii, Masanobu; Ichinose, Yuji; Kawanishi, Shunichi
Reza Kenkyu, 25(4), p.306 - 311, 1997/04
no abstracts in English
*; Seguchi, Tadao; Tabata, Yoneho*
Radiation Physics and Chemistry, 50(6), p.601 - 606, 1997/00
Times Cited Count:68 Percentile:96.84(Chemistry, Physical)no abstracts in English
*; Ikeda, Shigetoshi*; Seguchi, Tadao; Tabata, Yoneho*
Radiation Physics and Chemistry, 49(5), p.581 - 588, 1997/00
Times Cited Count:53 Percentile:95.04(Chemistry, Physical)no abstracts in English
Sakasai, Kaoru; Ara, Katsuyuki
Electromagnetic Phenomena Applied to Technology, 0, p.211 - 218, 1996/00
no abstracts in English
Tabata, Yoneho*; *; *; Seguchi, Tadao
Radiation Physics and Chemistry, 48(5), p.563 - 568, 1996/00
no abstracts in English
Abe, Hiroaki; Naramoto, Hiroshi; Kinoshita, Chiken*
Mater. Res. Soc. Symp. Proc., Vol. 373, 0, p.383 - 388, 1995/00
no abstracts in English
Mizuno, Makoto; Ohara, Yoshihiro; Watanabe, Kazuhiro; *
JAERI-M 93-214, 13 Pages, 1993/10
no abstracts in English
; *
Radiat.Eff.Lett., 86, p.43 - 46, 1985/00
no abstracts in English
*; ; *
Japanese Journal of Applied Physics, 20(8), p.L609 - L611, 1981/00
Times Cited Count:1 Percentile:6.58(Physics, Applied)no abstracts in English


 Si
Si

 alloy
alloy*; ; *; *; Furukawa, Kazuo
Journal of Nuclear Materials, 79(2), p.430 - 431, 1979/00
Times Cited Count:14no abstracts in English


 Si
Si

 alloy by the dense random packing model of differently sized compressible spheres
alloy by the dense random packing model of differently sized compressible spheres*; ; *; Furukawa, Kazuo
Phys.Status Solidi A, 51(2), p.325 - 332, 1979/00
Times Cited Count:3no abstracts in English